Suggested Citation
Marie-France Humblet1, Sébastien Vandeputte1, Adelin Albert, Christiane Gosset, Nathalie Kirschvink, Eric Haubruge, Fabienne Fecher-Bourgeois, Paul-Pierre Pastoret, and Claude Saegerman
Author affiliations: University of Liege, Liege, Belgium (M.-F. Humblet, S. Vandeputte, A. Albert, C. Gosset, F. Fecher-Bourgeois, C. Saegerman); University of Namur, Namur, Belgium (N. Kirschvink); University of Liege, Gembloux, Belgium (E. Haubruge); Fontin, Belgium (P.-P. Pastoret)
Abstract
To prioritize 100 animal diseases and zoonoses in Europe, we used a multicriteria decision-making procedure based on opinions of experts and evidence-based data. Forty international experts performed intracategory and intercategory weighting of 57 prioritization criteria. Two methods (deterministic with mean of each weight and probabilistic with distribution functions of weights by using Monte Carlo simulation) were used to calculate a score for each disease. Consecutive ranking was established. Few differences were observed between each method. Compared with previous prioritization methods, our procedure is evidence based, includes a range of fields and criteria while considering uncertainty, and will be useful for analyzing diseases that affect public health.
Agents that cause zoonotic diseases are infectious (transmissible) agents that not only are confined to 1 animal host but that also cause an infection (infestation) with or without clinical disease in several hosts, including humans (1). Nevertheless, all diseases affecting animals and humans are not strictly zoonotic but are qualified as common. Animals and humans generally contract infections from the same sources (soil, water, invertebrates, and plants). However, animals do not play an essential role in the life cycle of the etiologic agent but may contribute in various degrees to distribution and transmission of infections (2). According to the World Organisation for Animal Health (OIE), 75% of the emerging diseases originate in domestic or wild animals, which prompts close collaboration between animal health and public health authorities (www.oie.int/eng/edito/en_avr09.htm).
To achieve such a goal, the One Health strategy was recently developed to expand interdisciplinary collaborations and communications in all aspects of health care for humans, animals and the environment (www.onehealthinitiative.com/mission.php). Such collaborations are particularly evident when considering zoonoses. In the One Health concept, a new strategy for animal health was recently adopted by the European Union (3). Categorization of threats caused by animals is 1 of the pillars of this strategy. Such method aims to provide a tool for decisions in animal health issues for selecting disease-related threats that are worth being addressed by public policies, which is necessary if considering emerging infectious diseases. The method of disease prioritization has been defined as the “organization of listed diseases into a hierarchy, considering their respective impacts” (4).
The main objectives of a prioritization method are to optimize financial and human resources for the surveillance, prevention, control, and eradication of infectious diseases and to target surveillance for early detection of any emerging disease. Our method is based on widely used multicriteria analysis, which consists of listing criteria to assess pathogens, evaluating pathogens on the basis of these criteria (scores), determining the relative role (weight) of each criterion, and aggregating scores and weights of criteria into 1 global score per pathogen (5). The originality of the prioritization procedure is a reasoning based on opinions of multidisciplinary experts (weighting method) and on evidence-based data of targeted diseases (animal diseases, zoonoses, and transmissible diseases common to animals and humans).
Methods
Diseases
One hundred diseases were included in the prioritization exercise. Species targeted were food-producing animals: cattle, small ruminants, swine, horses, poultry (including water birds such as ducks and geese), lagomorphs, and wildlife (species common in western Europe). There were 86 diseases affecting the animal species under study and reportable to the OIE (www.oie.int/en/animal-health-in-the-world/oie-listed-diseases-2011/).
Influenza caused by highly pathogenic and low pathogenicity avian influenza viruses was considered separately. Twelve additional infectious diseases reported in Europe during September 2009 and September 2010 and reported to the International Society for Infectious Diseases (www.promedmail.org) (archives 20100215.0530, 20100803.2615, 20101227.4564, 20100729.2546, 20100330.0996, 20100111.0128, 20090912.3211, 20100620.2072, 20091217.4273, 20091004.3453, 20101106.4026, and 20100209.0442) were also included in the prioritization method: besnoitiosis, botulism, bluetongue (bluetongue virus serotype16), hantavirosis (Puumala virus), hepatitis E, influenza (H1N1), norovirus disease, European tick-borne encephalitis, Usutu virus disease, porcine post-weaning multisystemic wasting disease (circovirus), equine atypical myopathy, and disease caused by Escherichia coli O157:H7. Parafilariasis (Parafilaria bovicola) was also included because it is considered to be emerging in western Europe (6). Salmonellosis caused by Salmonella enterica serovar Enteritidis was considered because of its effect on public health and it is the most common serovar in the European Union (7). Foot-and-mouth disease (FMD) was included in the zoonotic/common category despite its low effect on public health and the limited number of human cases reported to date (8).
Prioritization Criteria
Five aspects of a pathogen were considered: epidemiology, prevention/control, effects on economy/trade, zoonotic characteristics, and effect on society. The prioritization criteria were established according to a review of previous priority settings (i.e., English Department for Environment, Food and Rural Affairs [DEFRA]) (http://archive.defra.gov.uk/foodfarm/farmanimal/diseases/vetsurveillance/strategy/programme/prioritisation.htm) (9–13) and principles of evidence-based medicine, which promotes collection, interpretation, and integration of valid, essential, and applicable scientific evidence (14). A total of 57 criteria were retained for the prioritization method and further submitted for opinions of experts. The distribution of criteria among 5 categories was 17 for epidemiology (EP), 8 for prevention/control (PC), 16 for economy/trade, 12 for public health (PH), and 4 for society (SO). The 57 criteria are summarized in Table 1. Strict definitions were given for each coefficient and criterion and are summarized in Table 2.
A particular classification of zoonoses based on interactions between host species is included as a criterion in the PH category. Type 1 diseases are those transmitted from wildlife to humans, type 1+ are transmitted from wildlife to humans with additional human-to-human transmission, type 2 are transmitted from wildlife to domestic animals and then to humans, and type 2+ are diseases transmitted from wildlife to domestic animals and then to humans, with further human-to-human transmission (15).
Coefficients of Criteria
For each criterion, a coefficient of 0–7 was assigned to each option (Table 2) according to its role, effect, or rate. Coefficients were correlated with severity: the more severe the effect, the higher the coefficient. For example, a case-fatality rate <1% has a coefficient of 1 and a case-fatality rate >90% has a coefficient of 7. For nonzoonotic agents, a coefficient of 0 was fixed for criteria included in the PH category.
For each disease, evidence-based information concerning the 57 criteria was obtained from different sources, including use of OIE and Iowa State University (Ames, IA, USA) fact sheets and consultation with websites of international organizations (OIE, World Health Organization, European Centre for Diseases Prevention and Control), and Centers for Disease Control and Prevention). Web site searches for peer-reviewed literature were conducted in PubMed and the Thomson Reuters (formerly Institute for Scientific Information) Web of Knowledge. Useful information was also obtained from scientific reference books (16,17). Searches enabled collection of ≈100% of information needed for the 57 criteria for the 100 diseases.
Multidisciplinary Panel of Experts
The main characteristic of the panel of experts consulted within the framework of the project was its multidisciplinarity. A total of 74 international experts were selected according to their field of expertise: veterinary and human epidemiologists, chief veterinary officers, economists, medical doctors, sociologists, and experts in public health and animal welfare.
Two tasks were assigned to the experts. First, they were asked to give their opinion on the pertinence of criteria proposed by indicating their degree of agreement. They were then asked to assign a score of 1 if they strongly disagreed with a criterion, 2 if they disagreed, 3 if they simply agreed, and 4 if they strongly agreed. In instances of strong disagreement, experts were asked to justify their decision and propose alternative options. Second, they were asked to weight criteria. Because all criteria do not have the same role in terms of risk and consequences within the same category, experts were thereafter asked to apply a Las Vegas method between the criteria according to their relative roles (or weights) (18). Because the number of criteria differs between categories, the number of points to distribute was proportional to the number of criteria per category: 90 for EP and PH, 60 for PC and EC, and 30 for SO. This method was necessary to prevent criteria classified as major by experts (in terms of points distributed) from receiving fewer points because they belonged to a category that included more criteria. Finally, 6 multicategory experts from international organizations were asked to apply the Las Vegas method for intercategory weighting by distributing 100 points between the 5 categories of criteria.
Weighting of Scores and Ranking According to Experts
After experts weighted the different criteria, an overall weighted score was calculated for each disease (19). To perform the ranking, we used an aggregation method that combined 2 types of weighting. First, intracategory weighting consisted of multiplying the coefficient (0–7) allocated to the criterion by the average of points (weight) distributed by the experts for that criterion. A global score for a category was obtained by summing the weighted scores obtained for each criterion. Second, multicategory experts performed intercategory weighting. The mean number of points allocated by these experts to each category of criteria (weight) was multiplied by the global score obtained for each category after the first weighting. The overall weighted score of each disease resulted from the summation of global scores obtained from the 5 categories, as shown in the equations OWS = Σcat (GSCj × IrWj) and GSCj = Σcrit (Ci × IaWi), where OWS = overall weighting score of a pathogen, GSCj = global score of a category of criteria, IrWj = intercategory weight for each category of criteria (average for deterministic method), Ci = initial coefficient per criterion, IaWi = intracategory weight for each criterion (average for deterministic method), Cat = categories of criteria, and Crit = criteria.
Uncertainty Analysis
Uncertainty of the weighting method was estimated by using a probabilistic method. All weights were converted into a function (Table 1) and computed by using @Risk software version 5.5 (Palisade Corporation, Ithaca, NY, USA). Functions were then combined through an aggregation method by using a Monte-Carlo simulation with 1,000 iterations to obtain a function of the overall weighted scores per disease with a 95% CI.
Classification of Diseases by Using Classification and Regression Tree Analysis
Different groups of roles were identified by using classification and regression tree (CART) (www.salford-systems.com) analysis with overall weighted scores per disease as input (probabilistic method). This widely used method developed by Breiman et al. (20) can be applied to analyze either categorical (classification) or continuous data (regression) (11,12,20,21). In this report, regression tree models were used as the target variable and disease role was the continuous variable (22). The aim of these models was to obtain subgroups with minimal within-variance (grouping diseases with a similar role) by using cross-validation (11,23). Default settings of the software described by Steinberg and Golowya were used to develop the regression tree (24).
Results
Expert Opinions for Criteria
The response rate of the 74 experts on the procedure was 54% (i.e., 40 replies). Profiles of the experts are shown in Table 3. Experts were classified according to the different categories of criteria as follows: 18 for EP, 16 for PC, 14 for EC, 10 for PH, and 13 for SO. Opinions of 6 cross-category experts who assessed all categories of criteria (multicategory experts) were also included individually in each category.
Opinions of experts on proposed criteria were taken into account to adapt the list of criteria. For example, in the EP category, 3 rates were proposed to experts: morbidity (illness), mortality, and case-fatality. The experts suggested deleting mortality rate because case-fatality rate better reflects the gravity of the disease. Some modifications (clarifications) were made to definitions of each criterion and its coefficients. The final database of criteria, their coefficients, and definitions used for prioritization are shown in Table 1. The relative weight of each category of criteria was 20 points (mean and median) for EP, 19 (mean) and 18 (median) for PC, 23 (mean) and 28 (median) for EC, 25 (mean and median) for PH, and 14 (mean and 15 (median) for SO.
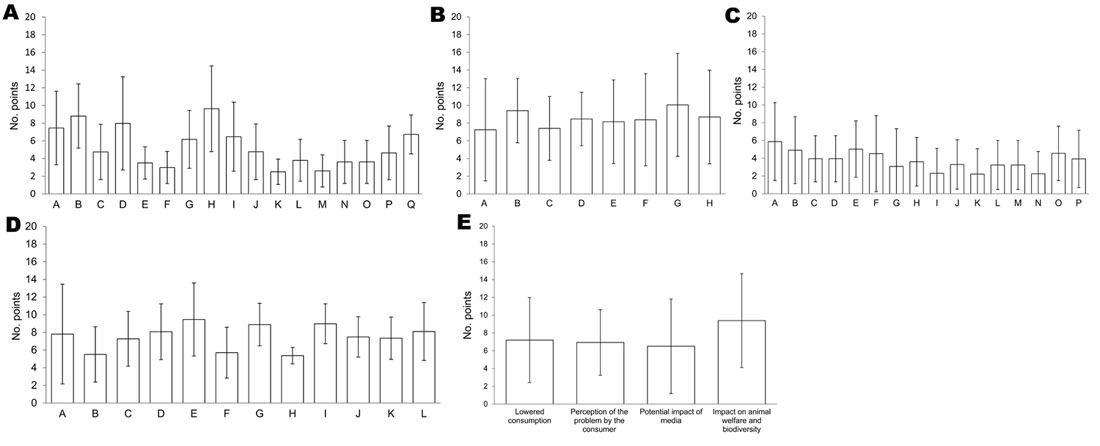
Figure 1
Figure 1. Weighting (mean no. points) of criteria for diseases of food-producing animals and zoonoses for 5 aspects of a pathogen proposed by experts, Europe. A) Epidemiology by 18 experts. A, illness rate;...
Public health was considered the major category of criteria in terms of prioritization. Weighting of criteria as proposed by experts is shown in Figure 1. Epizootic potential and case-fatality rate (%) were regarded as the 2 major epidemiologic indicators (Figure 1, panel A). Effectiveness of prevention and vaccination were weighted as the 2 major PC criteria (Figure 1, panel B). Loss of productivity and limitation of importation and exportation were the 2 major EC criteria (Figure 1, panel C). Case-fatality rate and epidemic potential were weighted as the 2 major PH criteria (Figure 1, panel D). Effect on animal welfare and biodiversity and lower consumption were the 2 major SO indicators (Figure 1, panel E). Nevertheless, within each category, differences between criteria were scarce. Conversely, the range in weights of each criterion was large, as shown by high SDs, which indicated variability among opinions of experts. To take variability into account, we used a probabilistic method to estimate the overall weighted score per disease.
Ranking of Diseases
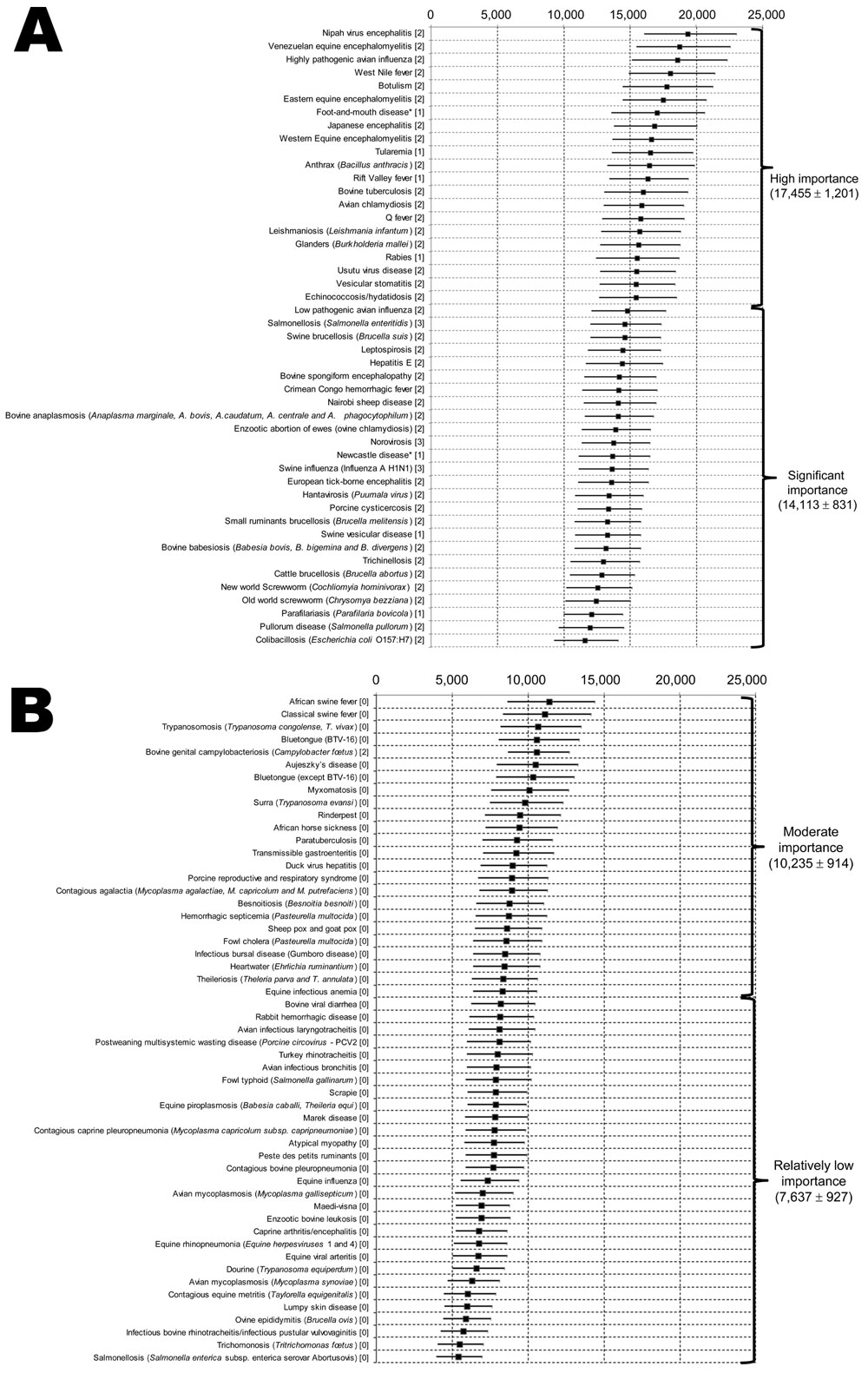
Figure 2
Figure 2. Classification and regression tree analysis showing grouping of diseases of food-producing animals and zoonoses into 4 subgroups by using overall weighted scores per disease as input, Europe. A) High importance and...
Final ranking of diseases according to their overall weighted scores and use of a probabilistic method is shown in Figure 2. Few differences were observed between deterministic (mean of each weight) and probabilistic methods (function of weights) (Pearson correlation coefficient 0.999; p<0.0001). This finding is probably associated with a few problems in subjective interpretation or dilution of individual discordances among the large number of experts.
FMD was considered as a zoonotic disease. However, FMD could be included in the nonzoonotic category. In such an instance, it would be the highest ranked nonzoonotic disease. Newcastle disease was included in the zoonotic/common category, which could be questioned because of its limited effect on PH. The top 5 ranked diseases were all zoonotic/common: Nipah virus encephalitis, Venezuelan equine encephalitis, influenza caused by highly pathogenic avian influenza virus, West Nile fever, and botulism.
Classification of Diseases by Using CART Analysis
Regression trees enabled identification of 4 groups of diseases. These diseases are shown in Figure 2.
Discussion
Prioritization of diseases has acquired major interest within the past few years, especially from a prevention point of view and in the sector of public health. Such a method is needed within the context of emerging diseases because it is not known how severe socioeconomic consequences of outbreaks will be. Our study included not only zoonoses, such as those reported by Cardoen et al. (11) and Havelaar et al. (12), but also transmissible diseases common to humans and animals and reportable animal diseases. The prioritization method was developed by an independent group to avoid any bias that could result from the influence of stakeholders as reported (25,26).
Several groups have proposed a prioritization method that considers different categories of criteria. Previous studies focused on a specific aspect of infectious diseases, such as the multicriteria analysis designed by Mourits et al., to support discussions on control measures (27). Conversely, the method developed by the French Agency for Food, Environmental and Occupational Health and Safety included major aspects of a disease (28) and also considered 2 rankings, 1 for animal health and 1 for human health. Nevertheless, our study provides a unique ranking that included both types of diseases.
The current method included more diseases compared with previous priority methods, such as those reported by DEFRA) (n = 25) or the European Commission (n = 46) (13). Krause et al. applied a Delphi method for collecting opinions, but only prioritized 85 zoonotic or common diseases (9,10). In our study, a Delphi method was initially planned but for time, rationale, and economic reasons, criteria were defined and established before being proposed to experts. Because our method includes qualitative and semiquantitative criteria, it is not completely numerically based, in contrast to the multicriteria analysis developed by Kurowicka et al. (5). Their method is applicable only for quantitative criteria and not always to all diseases in all contexts. Furthermore, they used a limited number of attributes for pathogens. Even if one relies on a quantitative method, which is less arbitrary than a semiquantitative method, the model developed by Havelaar et al. (12) is based on criteria reflecting the epidemiology and societal effect of zoonoses but does not include risk perception by the general public or diseases targeting animal species.
Our new method requires multidisciplinarity, which involves animal and human epidemiologists; chief veterinary officers; experts in agricultural economics, animal welfare, and biodiversity; and experts on societal aspects of diseases. Other prioritization methods often restricted their panel of experts to epidemiologists and infectious disease specialists (9,10).
The decision to start with the disease and not the animal species is in contrast to the method developed by Heffernan, who suggested that errors might be amplified throughout the weighting method (29). Nevertheless, by starting with the disease, the role of species is balanced by the EC category because the effect of different industries is taken into account. If an industry is not well developed in a specific area, the effect will be minimized. When the prioritization method is started with the species and its particular effect in the area/country, it makes the model applicable only in this specific area. However, if one starts with the disease and takes into account the economic role of the species in another category of criteria, the model can be applied anywhere.
Some methods applied a weighting system to criteria (DEFRA) (9,10,30) because it is not appropriate to consider all criteria on the same scale. For example, the human case-fatality rate should not be placed on the same scale as classification of zoonoses. Even if individual experts differed in their views on the relative role of various criteria and indicators, veterinary epidemiologists and experts in public health reached the conclusion that the epizootic/epidemic potential and case-fatality rate were the 2 major criteria in their respective category of expertise.
When one considers overall ranking of diseases, all top 20 diseases are zoonotic/common, which is expected because their global score involves the whole public health aspect. CART analysis also illustrates the correlation between PH and SO, which is not surprising because consumer behavior might be influenced when a zoonotic/common agent is involved (31). Nevertheless, CARTs might lead to slightly biased results in relation to variable selection: identification of distinct subgroups does not enable estimation of net effects of independent variables because subdivision of data into 2 groups is based on only 1 value of only 1 explanatory variable (32). In addition, bootstrap or jackknifing analysis would have been alternative ways to estimate the uncertainty.
The analysis can be applied only to the 100 diseases included in the model. Nevertheless, its predictive value is useful. The model we developed could be presented as generic and should not be confined to the 100 diseases included in the current application or to exotic diseases as with the method developed by French Agency for Food, Environmental and Occupational Health and Safety (28). At the beginning of the 21st century, a scientific team in the United Kingdom established a list of 1,415 pathogens that possibly affected humans (33). If added to the pathogens involved in animal diseases, all of these pathogens could also be added to the prioritization method, with a preliminary categorization step. As specified in the work performed under the aegis of the European Council, the prioritization exercise should be performed regularly as the epidemiologic situation of diseases constantly evolves: biotechnological improvements are constantly achieved in terms of vaccination, treatment, and diagnostic tests (30). In addition, elaboration of each criterion relied on evidence-based medicine through consultation with >1,800 scientific references (34; S. Vandeputte et al., unpub. data). The critical point of our method relies on the possible lack of independence between some criteria. Several of these criteria might be substantially dependent on each other. Although coefficients for ranges of illness and case-fatality rates were arbitrarily fixed, which may results in a loss of precision, they were accepted by experts. A Delphi method would have been more appropriate for reaching a consensus on the criteria to be used.
In conclusion, the current method is a generic tool applicable on different geographic scales in a variety of contexts because it is not restricted to well-defined field of actions. The standardization of criteria ensures transparency and reproducibility of the model in other context and for other diseases. It enables adaptations (vaccination becoming available, increased knowledge of a pathogen, viral mutations or genetic reassortments increasing host specificity). In the same view, the model could be applied to diseases affecting domestic (dogs, cats) pets or exotic pets (reptiles). Conversely, it could also be used with enzootic conditions to better retarget the surveillance system and readapt control measures worldwide.
Dr Humblet is a research scientist in the Unit of Research in Epidemiology and Risk Analysis at the University of Leige, Leige, Belgium. Her research interests are bovine tuberculosis and socioeconomic losses from emerging animal diseases and zoonoses.
Acknowledgment
This study was supported by the General Operational Direction, Agriculture, Natural Resources and Environment of the Walloon Region (D31–1225 convention).
References
Teufel P, Hammer P. Which zoonosis is it? [in Dutch]. Dtsch Tierarztl Wochenschr. 1999;106:311–8.PubMed
Acha PN, Szyfres B, eds. Preface of the first English edition. In: Zoonoses and communicable diseases common to man and animals. Vol. 1: Bacteriosis and mycoses [in French]. 3rd ed. Paris: World Organisation for Animal Health; 2005. p. ix.
European Commission. Health and Consumer Protection – Directorate-General, 2007. A new animal health strategy for the European Union (2007–2013) where “prevention is better than cure.” Communication from the Commission to the Council, the European Parliament, the European Economic and Social Committee, and the Committee of the Regions (COM 539 final) [cited 2011 Jan 6]. http://ec.europa.eu/food/animal/diseases/strategy/docs/animal_health_strategy_en.pdf .
Organisation for Animal Health. Phylum study: listing and categorisation of priority animal diseases, including those transmissible to humans—Mission Report 2010 [cited 2011 May 16]. http://www.oie.int/fileadmin/Home/eng/Support_to_OIE_Members/docs/ppt/OIE_study_priori-catego_mission_report.pdf .
Kurowicka D, Bucura C, Cooke R, Havelaar A. Probabilistic inversion in priority setting of emerging zoonoses. Risk Anal. 2010;30:715–23. DOIPubMed
Losson B, Saegerman C. First isolation of Parafilaria bovicola from clinically affected cattle in Belgium. Vet Rec. 2009;164:623–6. DOIPubMed
European Centre for Disease Prevention and Control. Annual epidemiological report on communicable diseases in Europe 2010. Stockholm: The Center [cited 2011 May 16]. http://www.ecdc.europa.eu/en/publications/Publications/1011_SUR_Annual_Epidemiological_Report_on_Communicable_Diseases_in_Europe.pdf
Berríos EP. Foot-and-mouth disease in human beings. A human case in Chile [in Spanish]. Rev Chilena Infectol. 2007;24:160–3.PubMed
Krause G; Working Group on Prioritization at the Robert Koch Institute. How can infectious diseases be prioritized in public health? A standardized prioritization scheme for discussion. EMBO Rep. 2008;9:S22–7. DOIPubMed
Krause G; Working Group on Prioritization at the Robert Koch Institute. Prioritization of infectious diseases in public health—call for comments. Euro Surveill. 2008;13:pii:18996. PubMed
Cardoen S, Van Huffel X, Berkvens D, Quoilin S, Ducoffre G, Saegerman C, Evidence-based semiquantitative methodology for prioritization of foodborne zoonoses. Foodborne Pathog Dis. 2009;6:1083–96. DOIPubMed
Havelaar AH, van Rosse F, Bucura C, Toetenel MA, Haagsma JA, Kurowicka D. Prioritizing emerging zoonoses in the Netherlands. PLoS ONE. 2010;5:e13965. DOIPubMed
Discontools project. Development of the most effective tools to control infectious animal diseases. 1Methods to the prioritization of diseases: a worldwide review of existing methodologies for health priority settings 2010 [cited 2011 Jan 5]. http://www.discontools.eu/documents/1232_Review%20of%20existing%20methodologies%20for%20priority%20settings%20-%20Draft2.pdf
Sackett DL, Rosenberg WM, Gray MJ, Haynes RB, Richardson WS. Evidence-based medicine: what it is and what it isn’t. BMJ. 1996;312:71–2. DOIPubMed
Kahn RE, Clouser DF, Richt JA. Emerging infections: a tribute to the one medicine, one concept. Zoonoses Public Health. 2009;56:407–28. DOIPubMed
Radostitis O, Gay GC, Hinchcliff KW, Constable PD, eds. Veterinary medicine. A textbook of the diseases of cattle, sheep, goats, pigs and horses. 10th ed. London: Saunders-Elsevier; 2007.
Kahn CM, Line S, eds. Merck Veterinary Manual. 10th ed. Whitehouse Station (NJ): Merck & Co., Inc.; 2010.
Gore SM. Biostatistics and the Medical Research Council. Medical Research Council News. 1987;35:19–20.
Department for Communities and Local Government, ed. Multi-criteria analysis: a manual. London: The Department; 2009 [cited 2011 Jan 7]. http://eprints.lse.ac.uk/12761/1/Multi-criteria_Analysis.pdf
Breiman L, Friedman JH, Olshen RA, Stone CJ, eds. Classification and regression trees. Pacific Grove (CA): Chapman and Hall Publisher; 1984.
VanEngelsdorp D, Speybroeck N, Evans JD, Nguyen BK, Mullin C, Frazier M, Weighing risk factors associated with bee colony collapse disorder by classification and regression tree analysis. J Econ Entomol. 2010;103:1517–23. DOIPubMed
Saegerman C, Speybroeck N, Vanopdenbosch E, Wilesmith JW, Berkvens D. Decision support tools for clinical diagnosis of disease in cows with suspected bovine spongiform encephalopathy. J Clin Microbiol. 2004;42:172–8. DOIPubMed
Speybroeck N, Berkvens D, Mfoukou-Ntsakala A, Aerts M, Hens N, Van Huylenbroeck G, Classification trees versus multinomial models in the analysis of urban farming systems in central Africa. Agric Syst. 2004;80:133–49. DOI
Steinberg D, Golowya M. CART—classification and regression trees. User’s guide 2007, San Diego: Salford Systems; 2007.
Scoones I, Wolmer W. Livestock, disease, trade and markets: policy choices for the livestock sector in Africa. Institute of Development Studies Working Paper 269. Brighton (UK): Institute of Development Studies, Brighton; 2006 [cited 2011 Jan 7] ftp://ftp.fao.org/docrep/nonfao/LEAD/af853e/af853e00.pdf
Perry B, Sones K. Science for development: poverty reduction through animal health. Science. 2007;315:333–4. DOIPubMed
Mourits MC, van Asseldonk MA, Huirne RB. Multi-criteria decision making to evaluate control strategies of contagious animal diseases. Prev Vet Med. 2010;96:201–10. DOIPubMed
French Agency for Food. Environmental and Occupational Health and Safety (ANSES). Methodology of prioritization of animal diseases; application to the example of pathogens exotic to metropolitan France [in French]. Report 2008-SA-0390, 2010 [cited 2011 Jan 6]. http://www.afssa.fr/Documents/SANT2008sa0390.pdf
Heffernan C. Panzootics and the poor: devising a global livestock disease prioritization framework for poverty alleviation. Rev Sci Tech. 2009;28:897–907.PubMed
Council of the European Union. EU animal health strategy: non-paper on prioritization of animal-related threats and biosecurity. Presented at: Second Meeting of CVO Working Party # 1. DG B I JR:ddc – 9536/08 ADD 1; 2008 May 22; Brussels. p. 1–8.
Frank C, Faber M, Askar M, Bernard H, Fruth A, Gilsdorf A, Large and ongoing outbreak of haemolytic uraemic syndrome, Germany, May 2011. Euro Surveill. 2011;16:pii:19878. PubMed
Speybroeck N. Classification and regression trees. Int J Public Health. 2011 Oct 21; [Epub ahead of print]. DOIPubMed
Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–9. DOIPubMed
Vandeweerd JM, Kirschvink N, Clegg P, Vandenput S, Gustin P, Saegerman C. Is evidence-based medicine so evident in veterinary research and practice? History, obstacles and perspectives. Vet J. 2012;191:28–34. DOIPubMed
Fonte: CDC
Neste Blog fazemos: 1- Atualização sobre a ocorrência de doenças de importância em Veterinária e em Saúde Pública em todo o mundo. 2- Troca de informações sobre: Doenças Infecciosas, Zoonoses, Saneamento Ambiental, Defesa Sanitária Animal (Legislação e Programas Sanitários do Ministério da Agricultura) e demais assuntos relacionados à sanidade e Saúde Pública. Este blog se destina a discutir a saúde animal dentro dos seus mais variados aspectos.
Assinar:
Postar comentários (Atom)
Hi, this blog is really amazing and provide me answers to all my questions. This is really informative and I will for sure refer my friends the same. Thanks.
ResponderExcluirHi!!! We're happy for you!!!! We´re working hard to offer good information to you. Share our blog with your friends. Thanks...
Excluir